Workplan
Our work plan is structured according to a basic life-cycle model for guidedlines and protocols, as depicted below, with each of the steps in this life-cycle corresponding to one of our work packages.
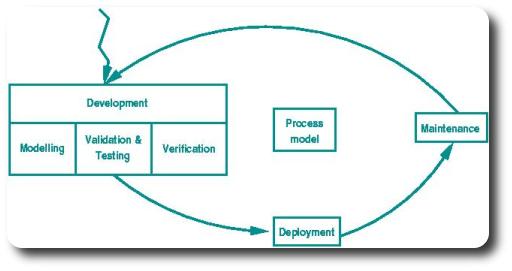
WP 1: Process model
Objectives
To elaborate a new model for a rigorous guideline development process.
Description of work
In this work package we will study the suitability of existing process model languages for the modelling of the design process of medical guidelines, and we will make a choice for one of these languages. Afterwards, we will concentrate on the study of the activities within the current guideline development process, and on the elaboration of a new proposal allowing for a smooth integration of our formal techniques & tools.
Deliverables
- D1.3 New model of guideline development process (report)
Milestones
- M1.1 Choice of process model language
- M1.2 Study of existing practice in guideline development process
WP 2: Development-Modelling part
Objectives
Select a reference guideline on which to apply our formal tecniques & tools.
Define adequate representation formats for the development of (formal or informal) medical guidelines, and apply them to the reference guideline.
Facilitate the modelling of medical guidelines by means of tool support and design patterns.
Description of work
In this work package
- we will select and motivate the guideline that will be used as reference throughout the project
- we will specify and/or refine different representation formats adequate to the formalisation of medical guidelines, and we will use them to formalise the reference guideline
- we will develop tools to support the process of transformation of medical guidelines into their formal counterparts; and, finally
- we will analyse recurrent patterns in medical guidelines, with potentials for reuse.
Deliverables
- D2.2a Specification of formats of intermediate, Asbru and KIV representations (report)
- D2.2bcd Models of selected guideline in intermediate, Asbru and KIV representations (report)
- D2.3a Mark-up & editing tool (prototype)
- D2.3b Asbru-to-KIV translator (prototype)
- D2.4 Visualisation tool (prototype)
- D2.5Library of design patterns for guidelines (report)
Milestones
- M2.1 Motivation and selection of reference guideline
- M2.2a Specification of formats
- M2.2b Model of selected guideline in intermediate representation
- M2.2c Model of selected guideline in Asbru
- M2.2d Model of selected guideline in KIV
WP 3: Development-Validation / Testing part
Objectives
Facilitate the validation/testing of medical guidelines with the support of an interpreter.
Study techniques adequate to the validation/testing of medical guidelines, and apply them to the reference guideline.
Description of work
In this work package
- we will work on the implementation of a tool for the interpretation of Asbru guidelines that allows their interactive validation by means of on-line simulation tests; and
- we will study the state-of-the-art in testing strategies, to determine the more suitable ones for medical guidelines, and we will apply them to check the Asbru model of the reference guideline
Deliverables
- D3.1 Asbru interpreter (prototype)
- D3.2b Testing of selected guideline (report)
Milestones
- M3.2a Testing strategics for medical guidelines
WP 4: Development-Verification part
Objectives
Facilitate the rigorous verification of medical guidelines using theorem proving and model-checking.
Apply these techniques to the verification of selected properties in the reference guideline.
Description of work
In this work package we will work on
- the definition of verification properties in parallel with guideline development
- the extensions to the semantics needed for the formal verification of guidelines in full Asbru
- the necessary improvements to the KIV verification system; and
- the application of the improved KIV system and of a model-checker to the verification of a selection of the previously defined properties.
Deliverables
- D4.2b Improved verification system (prototype)
- D4.2c Formal verification of selected guideline properties (report)
- D4.3 Model-checking of selected guideline properties (report)
Milestones
- M4.1 Specification of guideline properties & indicators
- M4.2a Extended semantics
WP 5: Deployment
Objectives
Provide some basic support for the deployment of medical guidelines, for both the refinement of protocols from guidelines and the critiquing based on guidelines.
Description of work
In this work package
- we will carry out a case-study of protocol development from a concrete guideline; and
- we will use formal methods (model-checking) to critique the care given to a patient using the recommendations issued from a particular guideline.
Deliverables
- D5.1 Case-study in transformations for protocol development from guidelines (report)
- D5.2 How to use model-checking for critiquing using medical guidelines (report)
WP 6: Maintenance
Objectives
Facilitate the validation and verification of continuously evolving medical guidelines.
Description of work
This work package will focus on the development (and integration in the KIV system) of techniques to facilitate the validation and verification of evolving guidelines, such as the reuse and replay of proofs, generation of counterexamples, and change management.
Deliverables
- D6 Living proofs (prototype)


